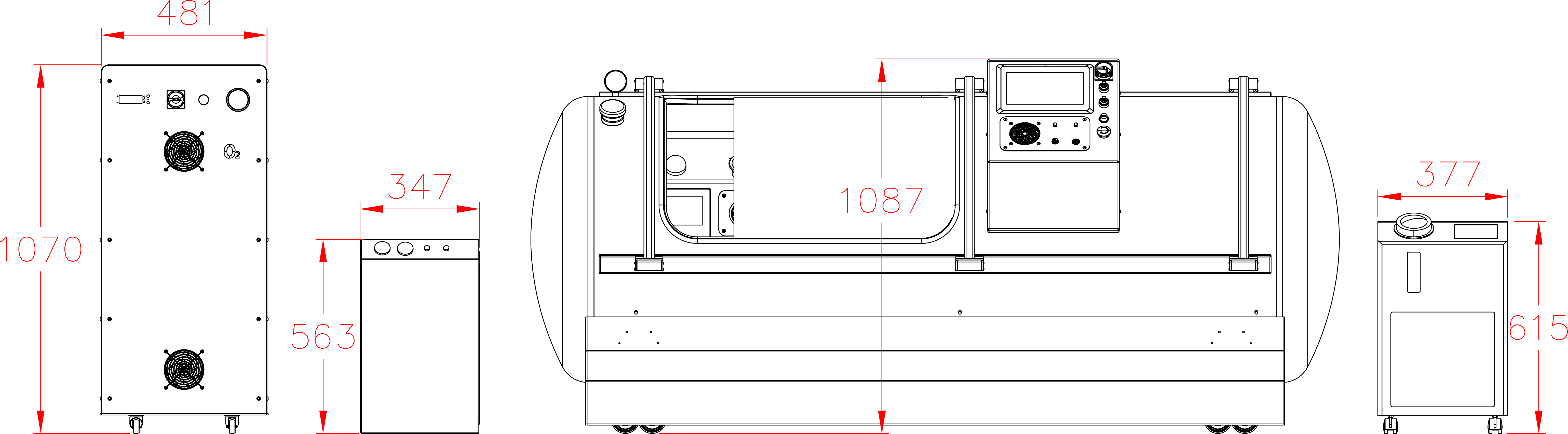
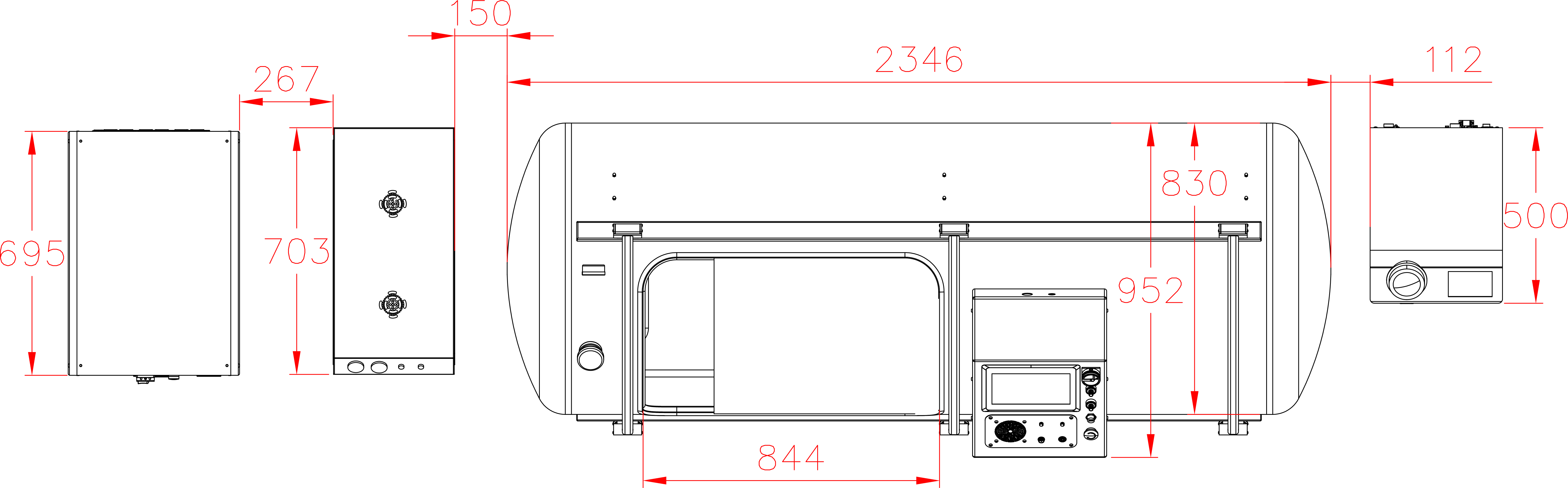
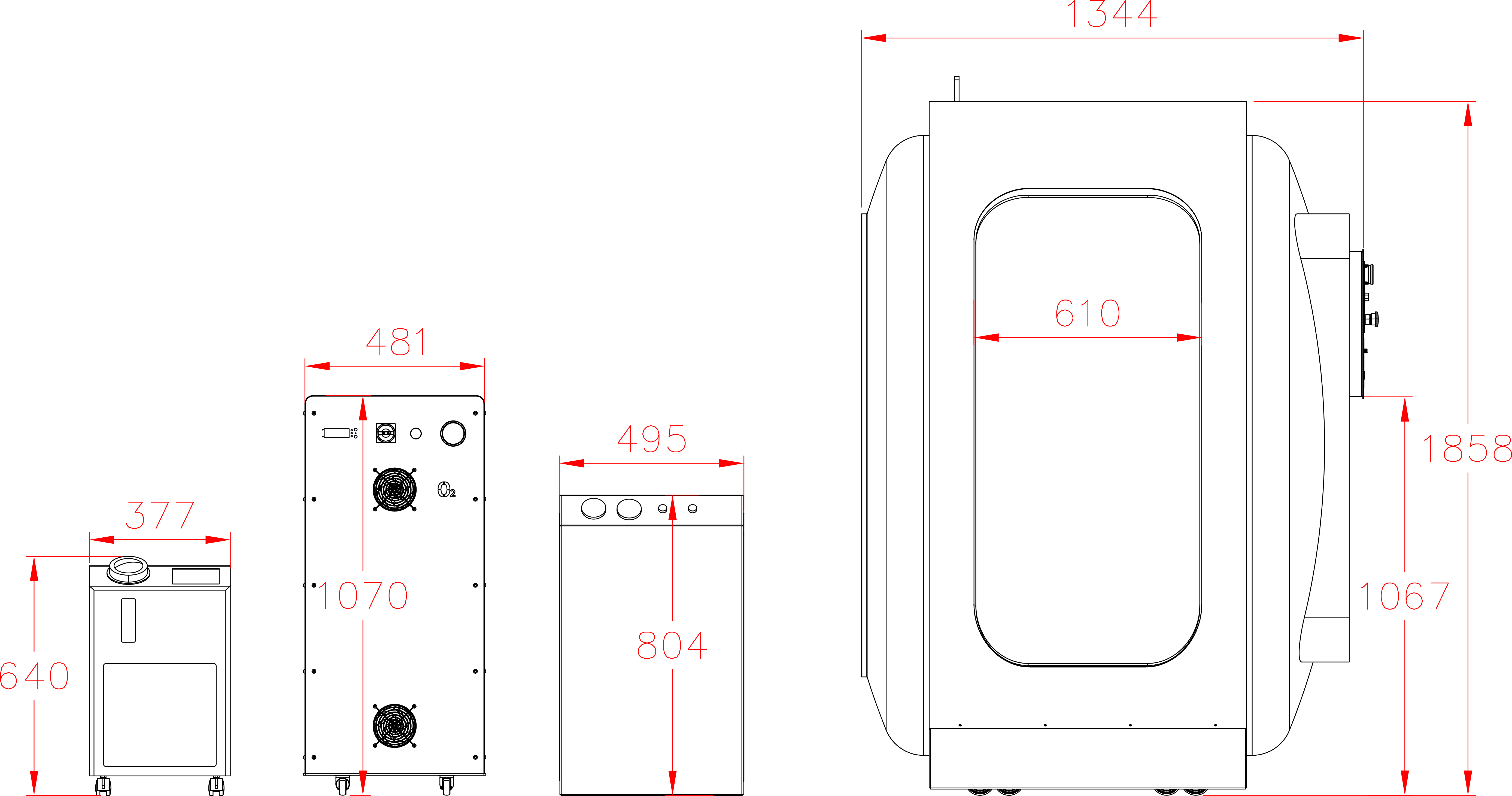
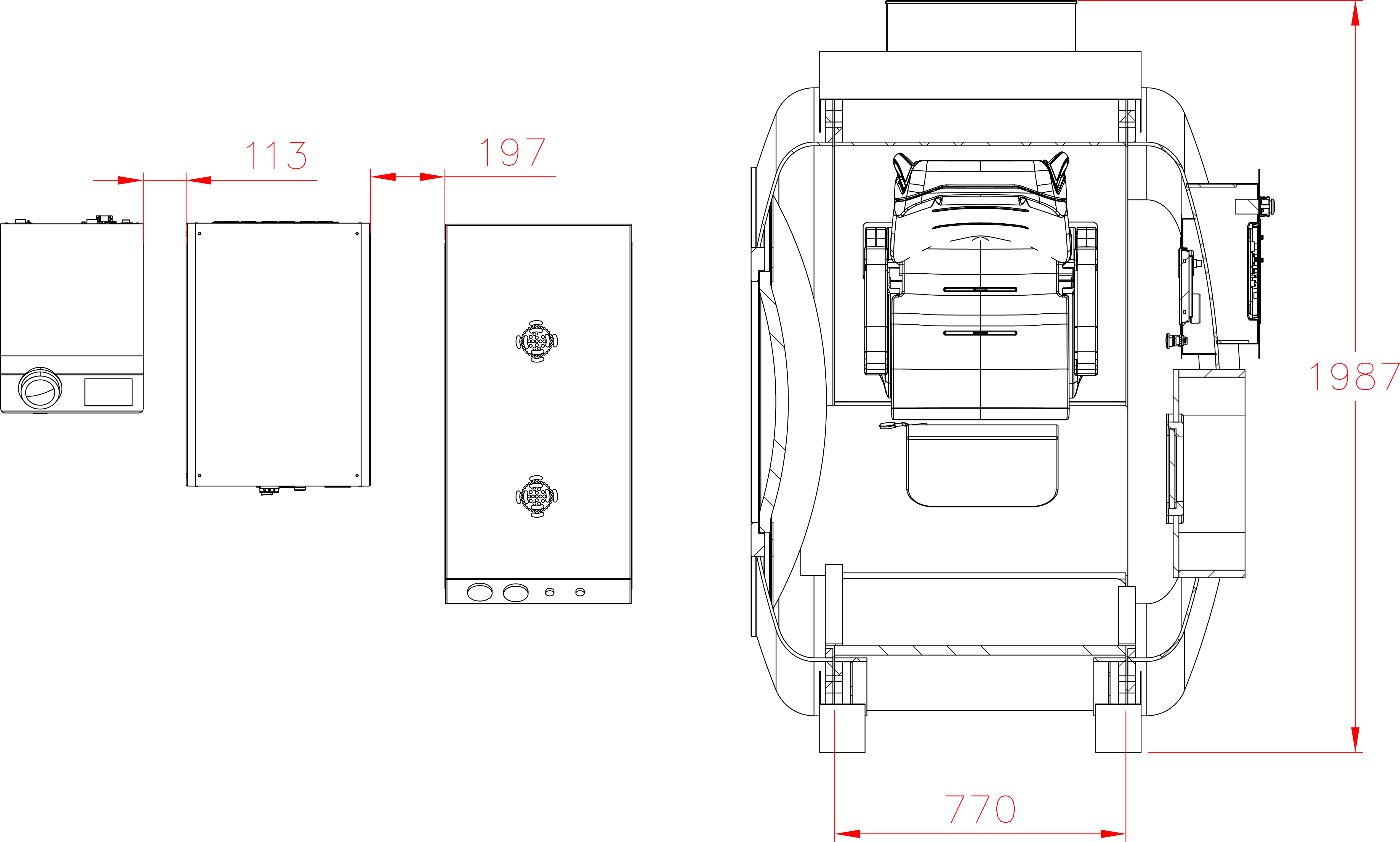
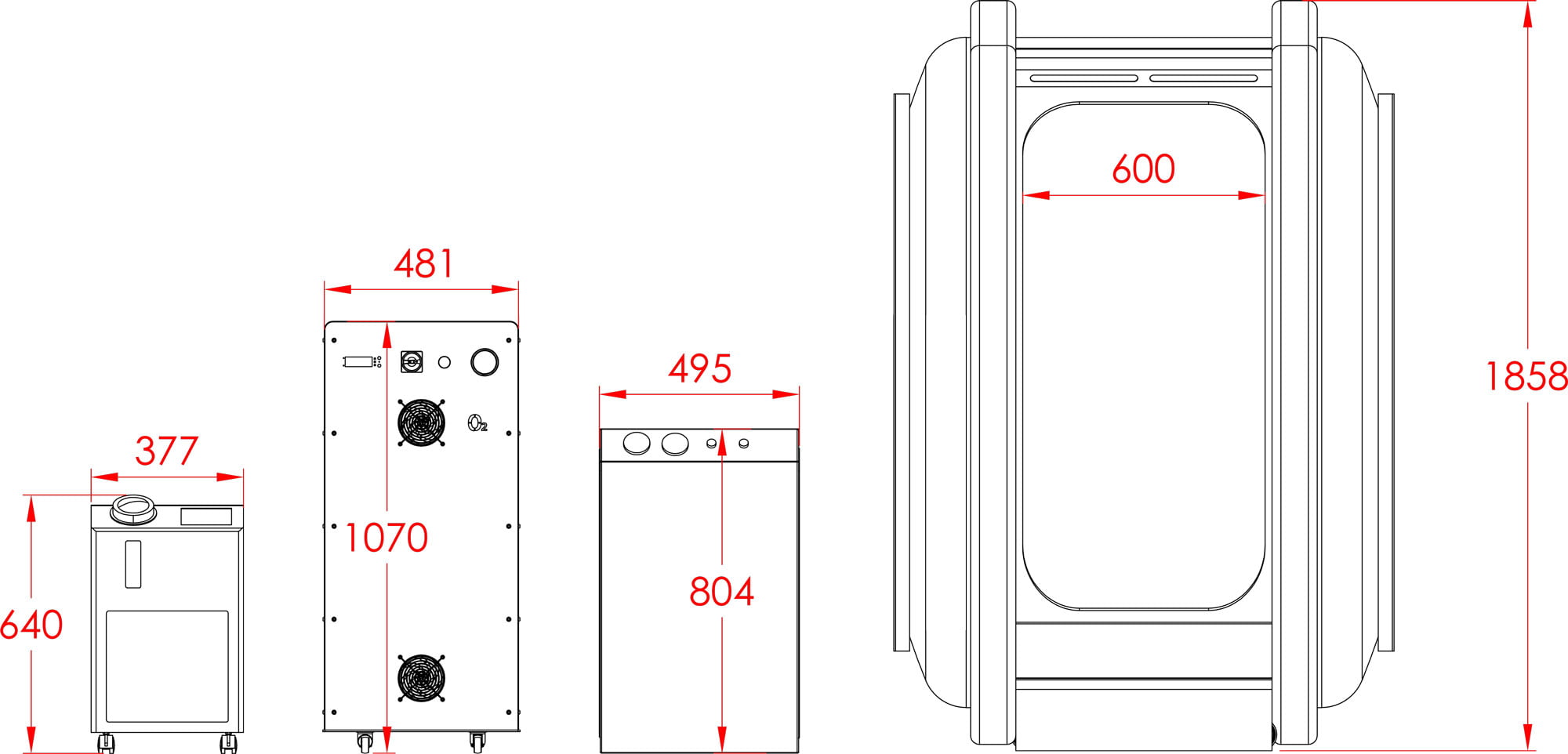
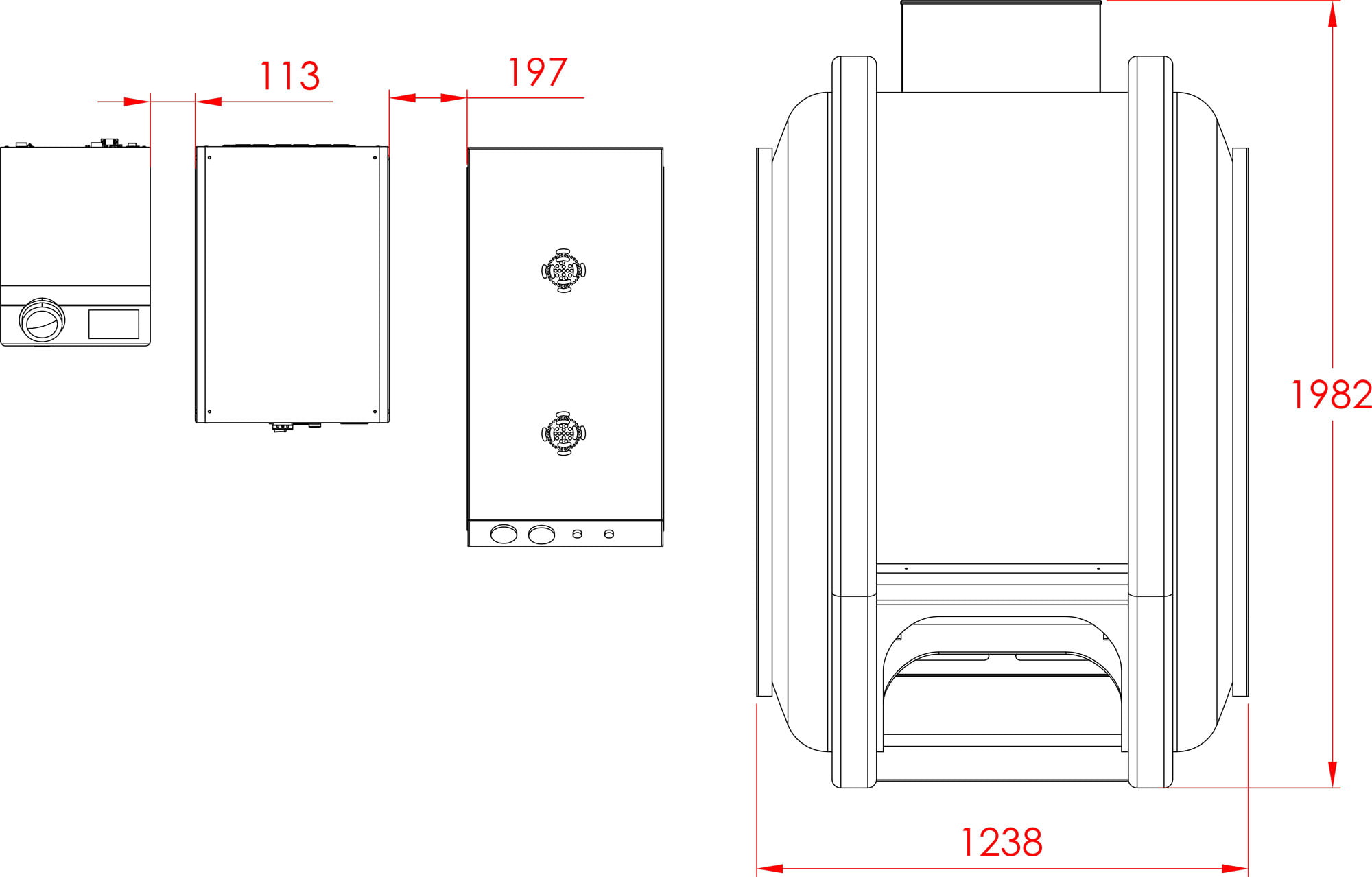
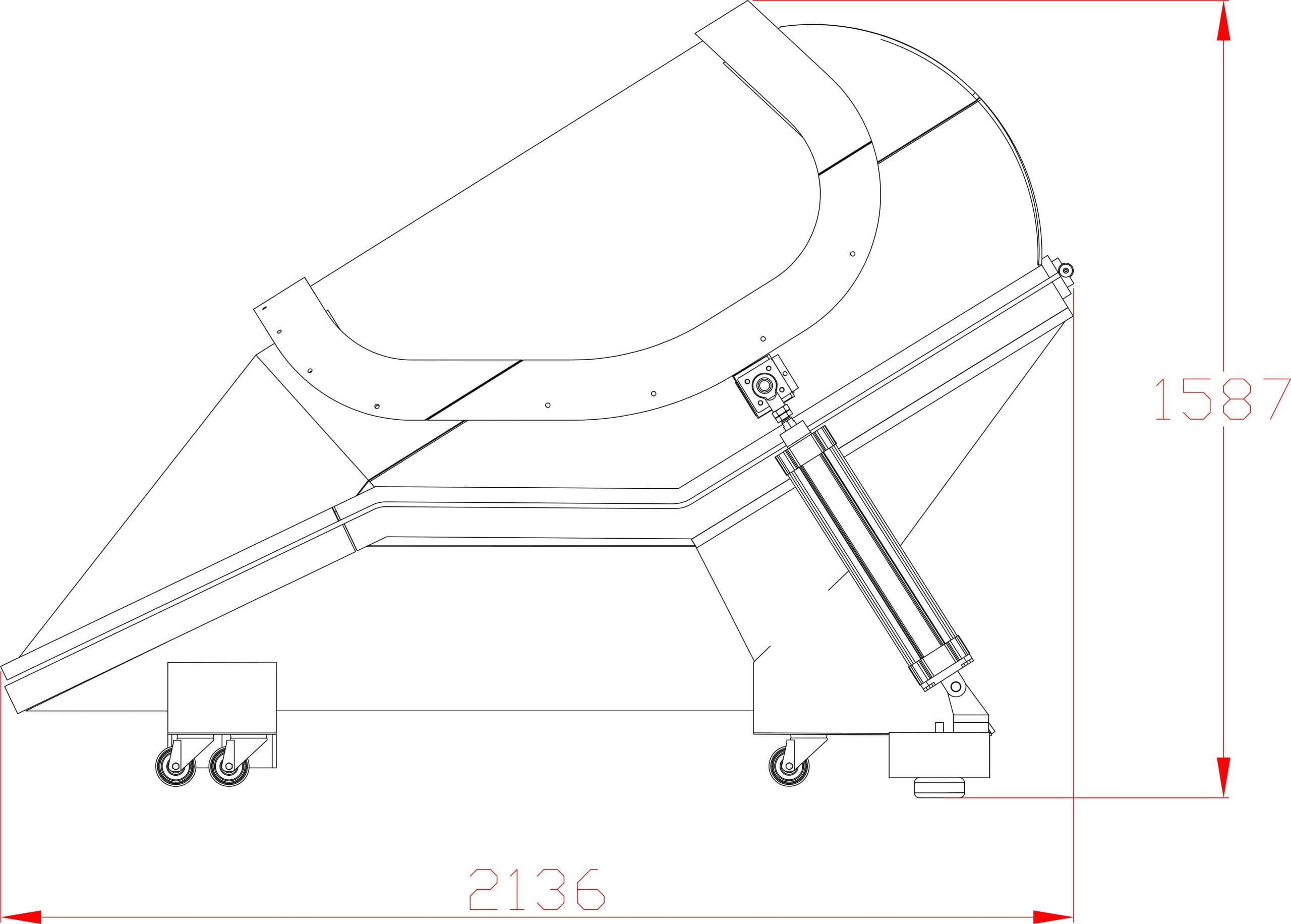
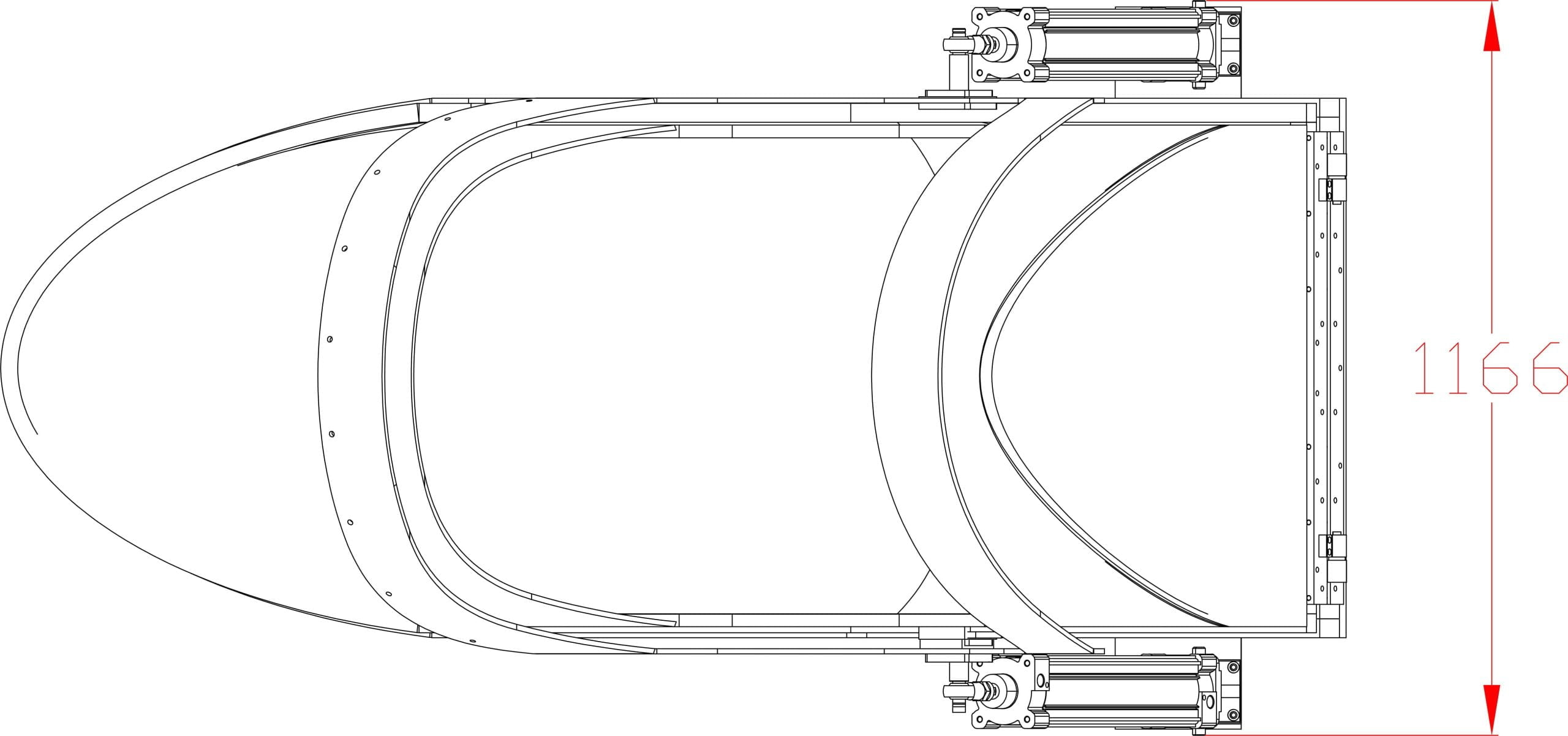
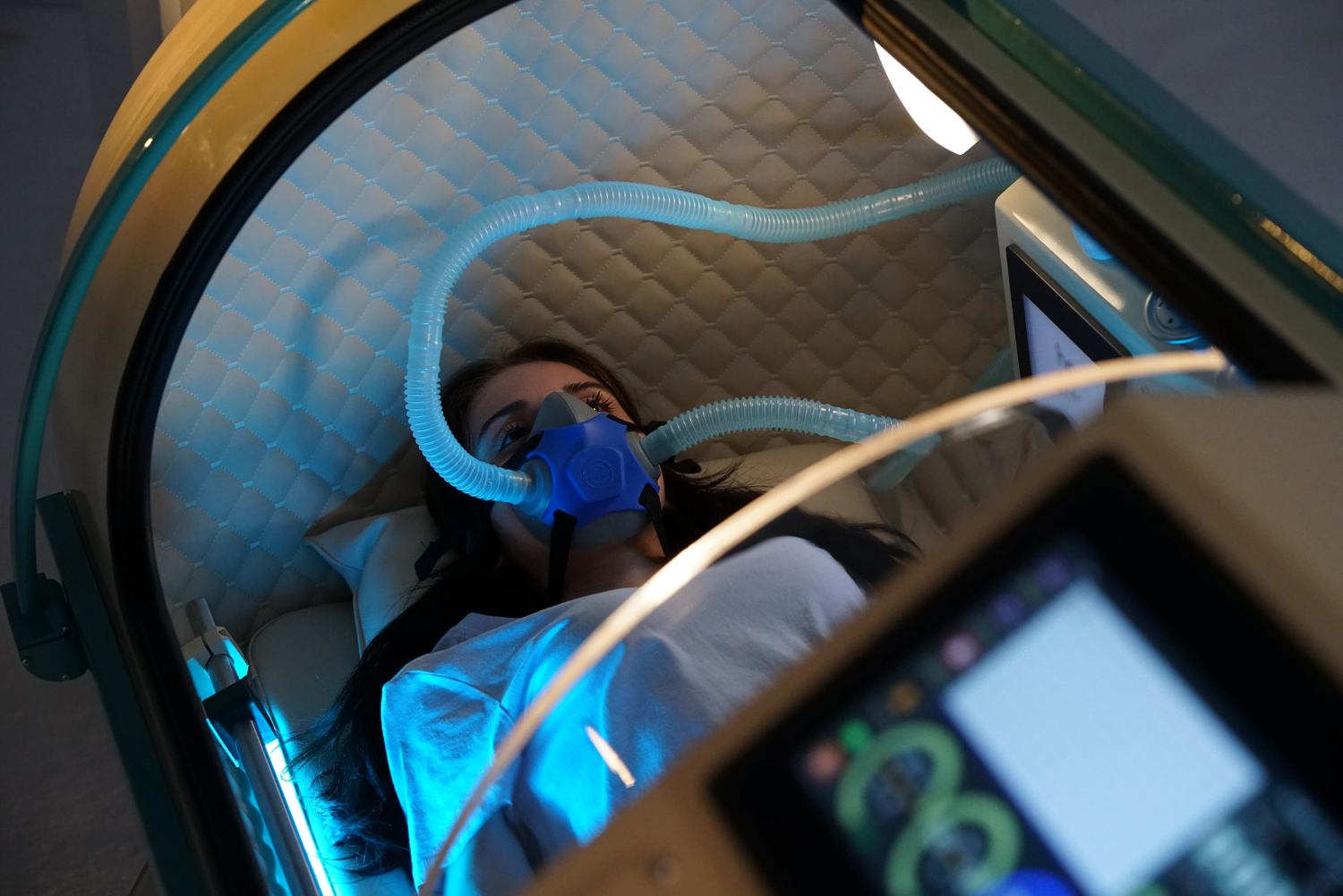
Our Total Quality Management system includes all aspects across design, manufacturing, testing, functionality, and customer service to ensure our hyperbaric oxygen systems are effective in therapy, but also safe, reliable, and accessible for patients.
Certifications and Total Quality Management for hyperbaric chamber manufacturing
Quality testing
Our quality control team coordinates the manufacturing, in-house testing, including the third-party testing process according to international regulations, procedures, and criteria for the certification of medical devices for medical HBOT & wellness applications.
Safety and compliance
Safety standards and certifications play a vital role in guaranteeing the quality and reliability of hyperbaric chambers used for hyperbaric oxygen therapy (HBOT).
Certifications
HPO TECH hyperbaric chambers comply with a wide range of international regulatory standards to ensure safety, quality, and operational excellence.
These certifications are categorized based on their focus, covering medical device compliance, pressure vessel safety, product and occupational safety, quality management, and regional market approvals.
Medical Device Regulations

93/42/EEC – Medical Devices Directive (MDD)
This European Union directive ensures that HPO TECH hyperbaric chambers meet essential safety and performance requirements for medical devices. Compliance with MDD guarantees that the chambers are suitable for therapeutic use within healthcare settings.

Medical Devices Directive (MDR) – In process of certification
The company is transitioning from the MDD (Medical Devices Directive) certification with the notified body UBED to the new MDR (Medical Device Regulation) framework with the notified body ICIM.
A letter of continuance from ICIM, along with a contract outlining the transition process, ensures regulatory compliance during the certification shift. This transition reflects our commitment to aligning with the latest EU medical device regulations for enhanced safety and performance standards.

UKCA – UK Conformity Assessed
Applicable to the UK market, this certification confirms that our chambers meet post-Brexit regulatory requirements for medical devices, ensuring compliance with safety, performance, and environmental protection standards.

SFDA – Saudi Food and Drug Authority
This certification allows HPO TECH hyperbaric chambers to be marketed and used in Saudi Arabia by verifying compliance with safety, quality, and efficacy requirements for medical devices.

DOH – Department of Health Abu Dhabi
A certification required for medical devices sold and used in Abu Dhabi, confirming compliance with regional health and safety standards for patient care.

Medsafe, New Zealand
New Zealand’s medical device regulatory body ensures that HPO TECH chambers meet national healthcare standards for safety and effectiveness.
Pressure vessel and safety regulations

ASME PVHO – Pressure Vessels for Human Occupancy
This American Society of Mechanical Engineers (ASME) standard ensures that our hyperbaric chambers are designed and built to safely accommodate human occupants under pressurized conditions, reducing risks associated with high-pressure environments.

PED – Pressure Equipment Directive
An European standard that regulates the design, manufacturing, and testing of pressurized equipment, ensuring the hyperbaric chambers can safely handle the pressures required for hyperbaric therapy.

Turk Lloydu – Hydrostatic Testing and Conformity Assessment:
Turk Lloydu provides certification for pressure testing and structural integrity of hyperbaric chambers, verifying their safety and compliance with international hydrostatic standards.
Product and occupational safety standards

2006/42/EC – Machinery Directive
This directive ensures the mechanical safety of hyperbaric chambers, covering aspects such as moving parts, emergency stops, and user interfaces to protect operators and patients from mechanical hazards.

NFPA – National Fire Protection Association (USA)
Establishes fire safety guidelines for hyperbaric chambers, ensuring compliance with strict fire prevention and suppression standards within medical environments.

ANEP 26 – Ergonomic Military Standards
This ergonomic standard establishes general human engineering design criteria for military systems, subsystems, equipment, and facilities

2001/95/EC – General Product Safety Directive
This certification allows HPO TECH hyperbaric chambers to be marketed and used in Saudi Arabia by verifying compliance with safety, quality, and efficacy requirements for medical devices.
Quality and management systems certifications

ISO 13485 – Medical Devices Quality Management System
This certification confirms that HPO TECH adheres to internationally recognized standards for medical device manufacturing, ensuring quality control throughout the product lifecycle.

ISO 9001 – Quality Management System
This directive ensures the mechanical safety of hyperbaric chambers, covering aspects such as moving parts, emergency stops, and user interfaces to protect operators and patients from mechanical hazards.

ISO 45001 – Occupational Health and Safety Management System:
Ensures that our chambers are manufactured under a management system that prioritizes employee safety and health in the workplace.

ISO 14001 – Environmental Management System
Confirms that the company follows environmental best practices in its manufacturing processes, reducing ecological impact and ensuring sustainability.

ISO 27001 – Information Security Management System
Ensures that the company protects sensitive patient and operational data through strict information security management protocols.

Quality control
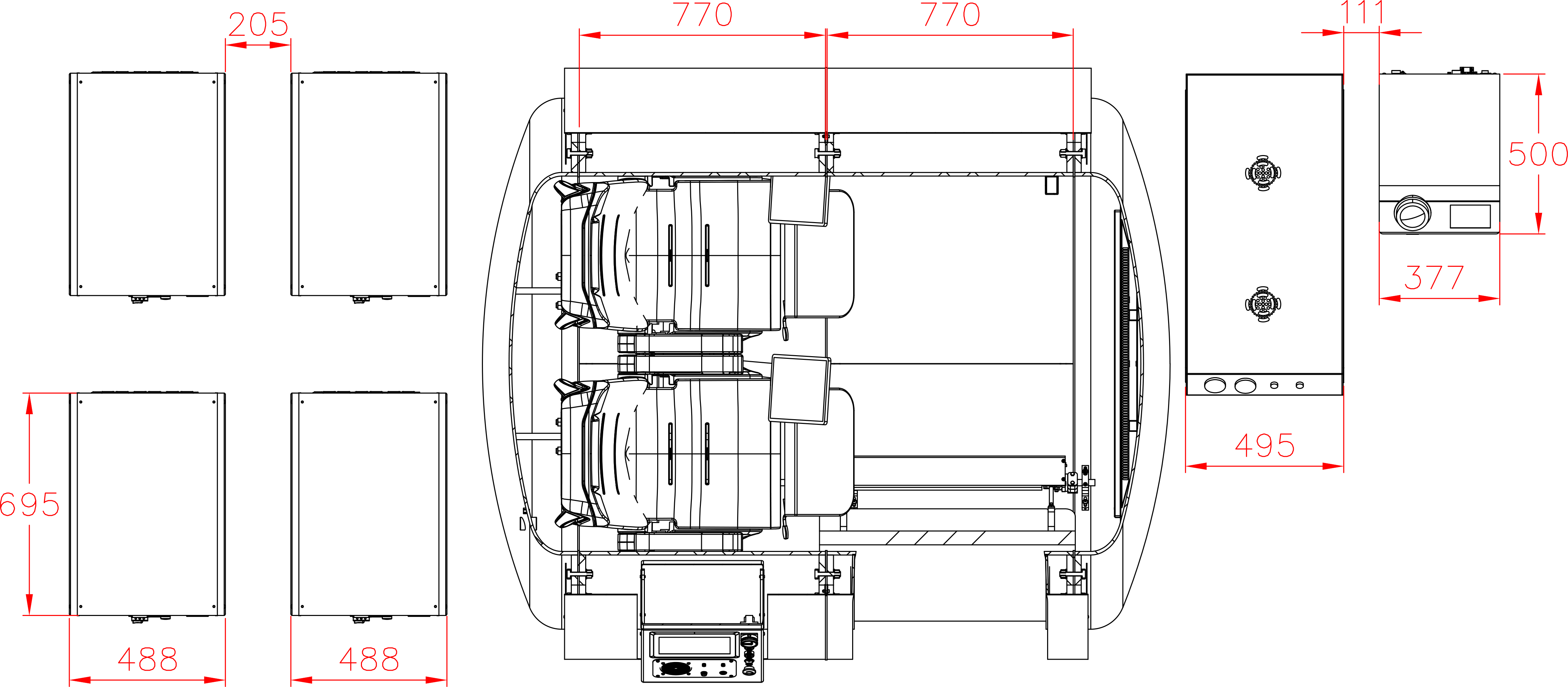
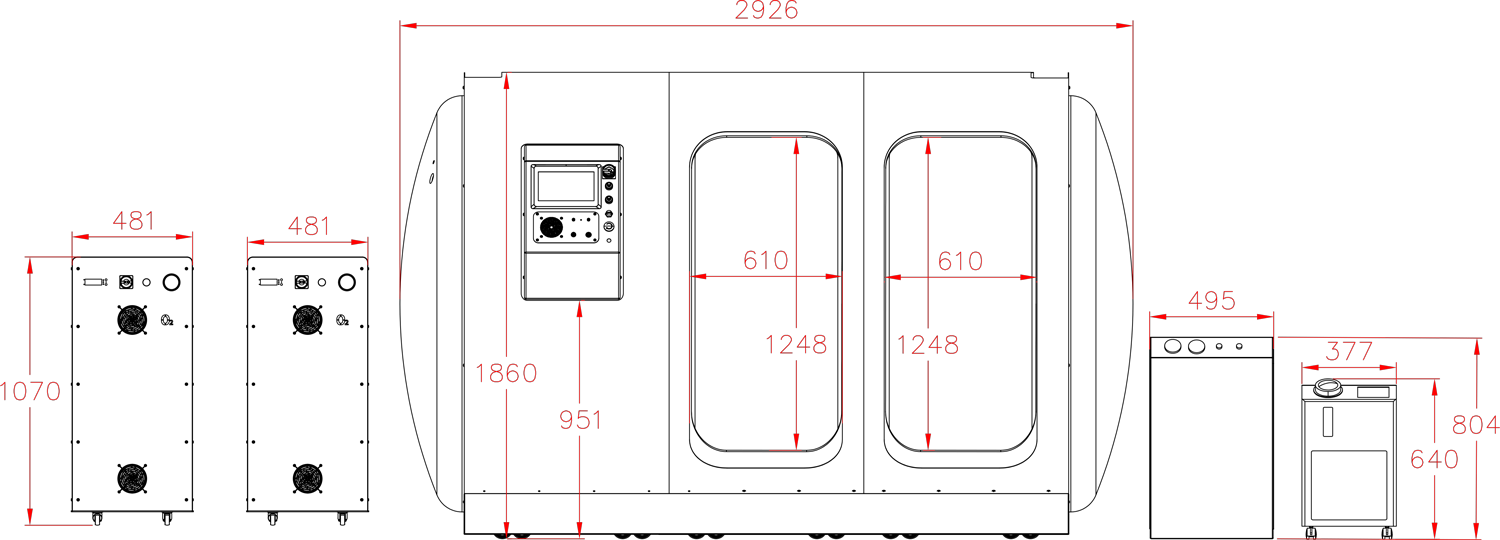
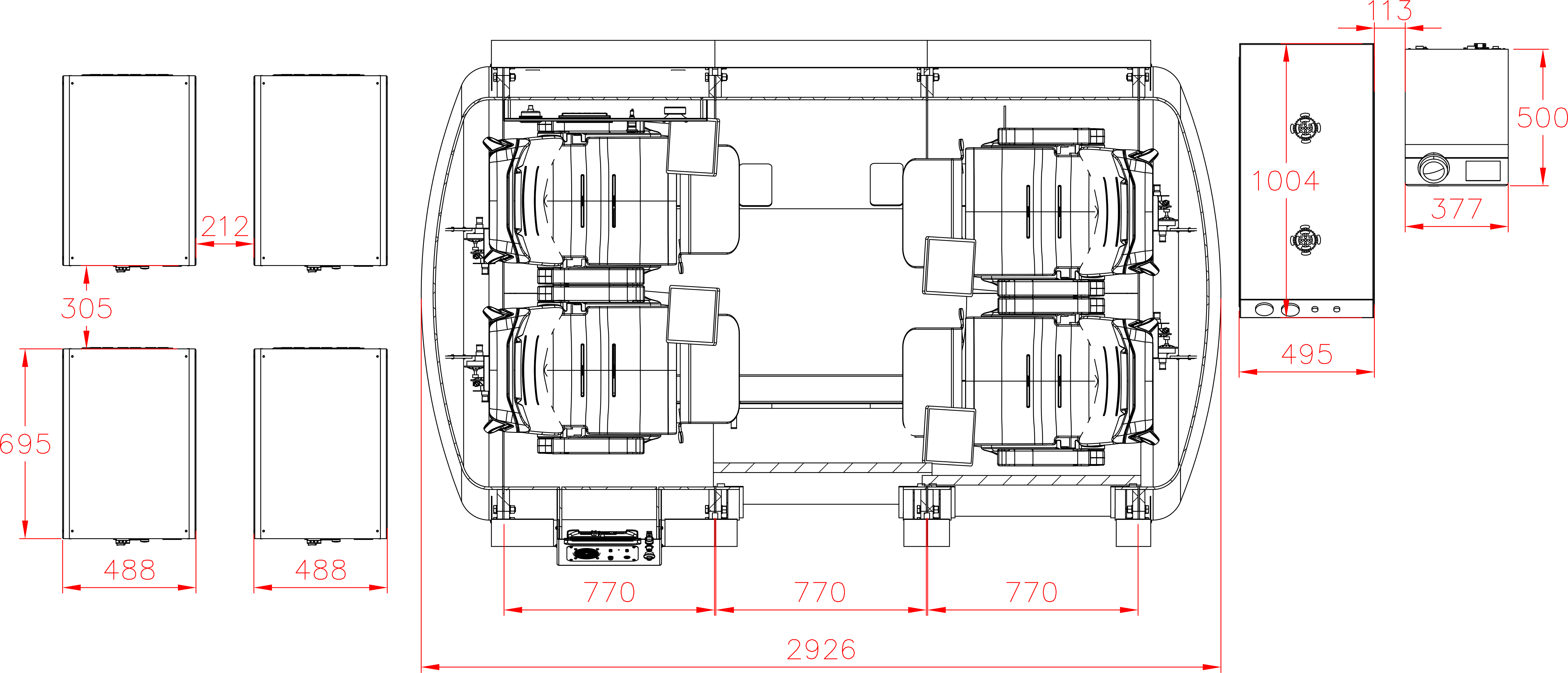
Each hyperbaric chamber is inspected by Türk Loydu after manufacturing in compliance with the management systems standards in quality, environmental safety, occupational health and safety, energy management, and structural integrity.
Hydrostatic Testing for Hyperbaric Chambers
Hydrostatic testing plays a critical role in guaranteeing the safety and structural integrity of hyperbaric chambers. This non-destructive quality control procedure involves pressurizing the chamber with water (deionized water to avoid corrosion) to a level exceeding its maximum operating pressure.
By simulating the real-world pressure conditions of HBOT sessions, hydrostatic testing helps identify any weaknesses in the chamber’s materials, welds, or seals before they pose a risk during therapy. This important quality control step ensures patients can experience the benefits of HBOT with confidence, knowing the chamber has undergone rigorous testing for leaks and structural integrity.